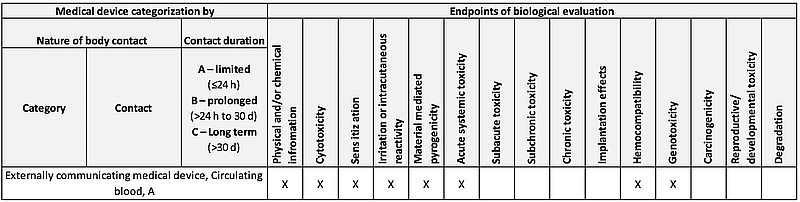
Towards a Logic-Based Extension of a Relational Software Tool for Coherent Technical Documentation of Medical Devices - In Compliance Magazine

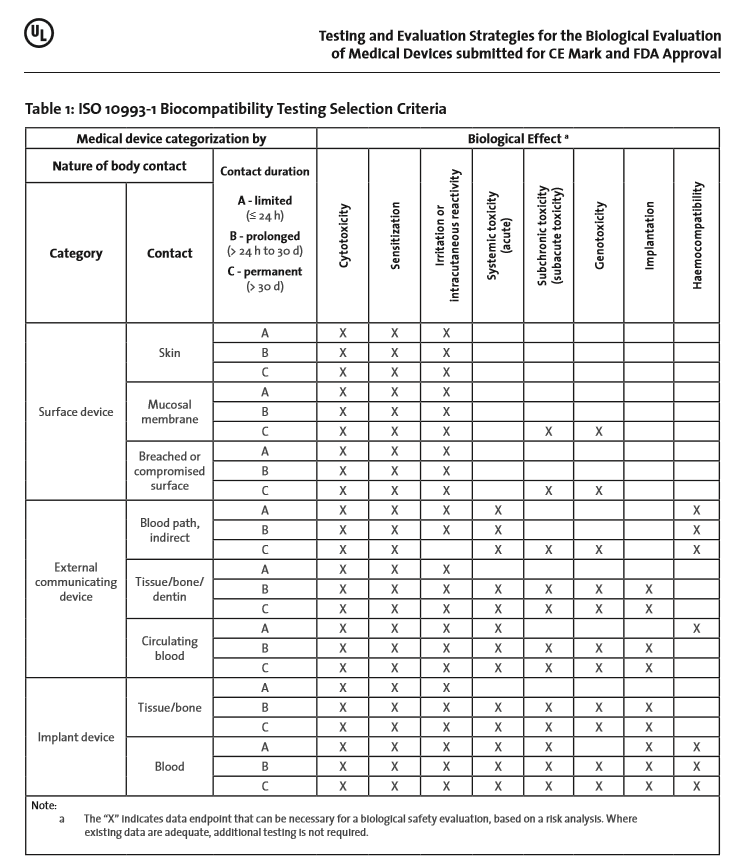
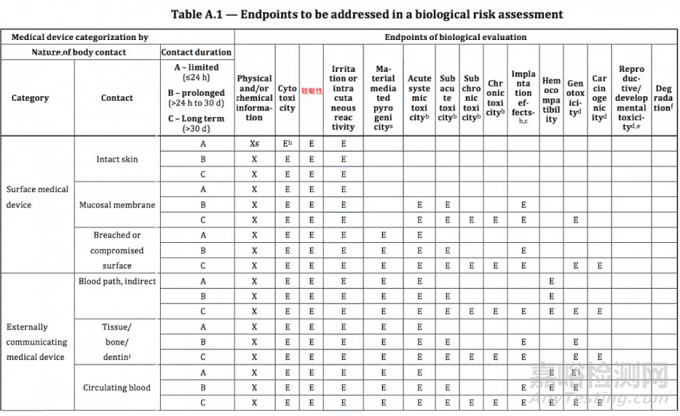
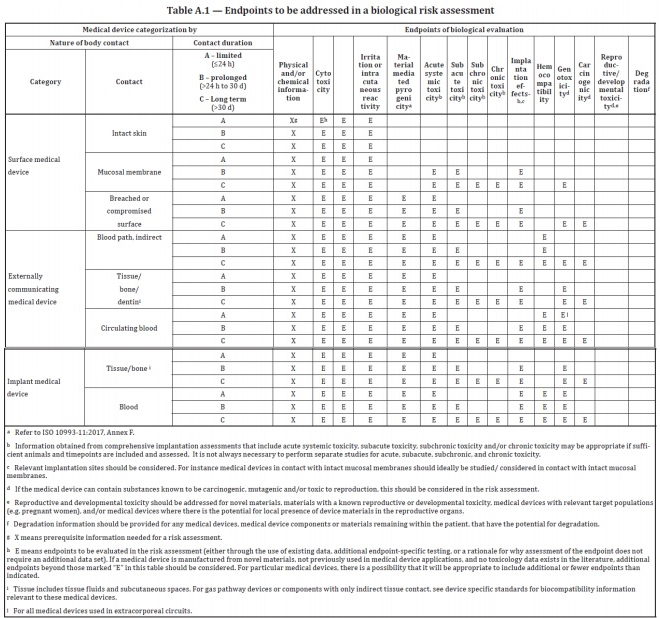
ISO 10993-1:2018(en), Biological evaluation of medical devices — Part 1: Evaluation and testing within a risk management process

Understanding Food-Grade Vs. Biocompatibility For Medical Device Materials | Medical Product Outsourcing

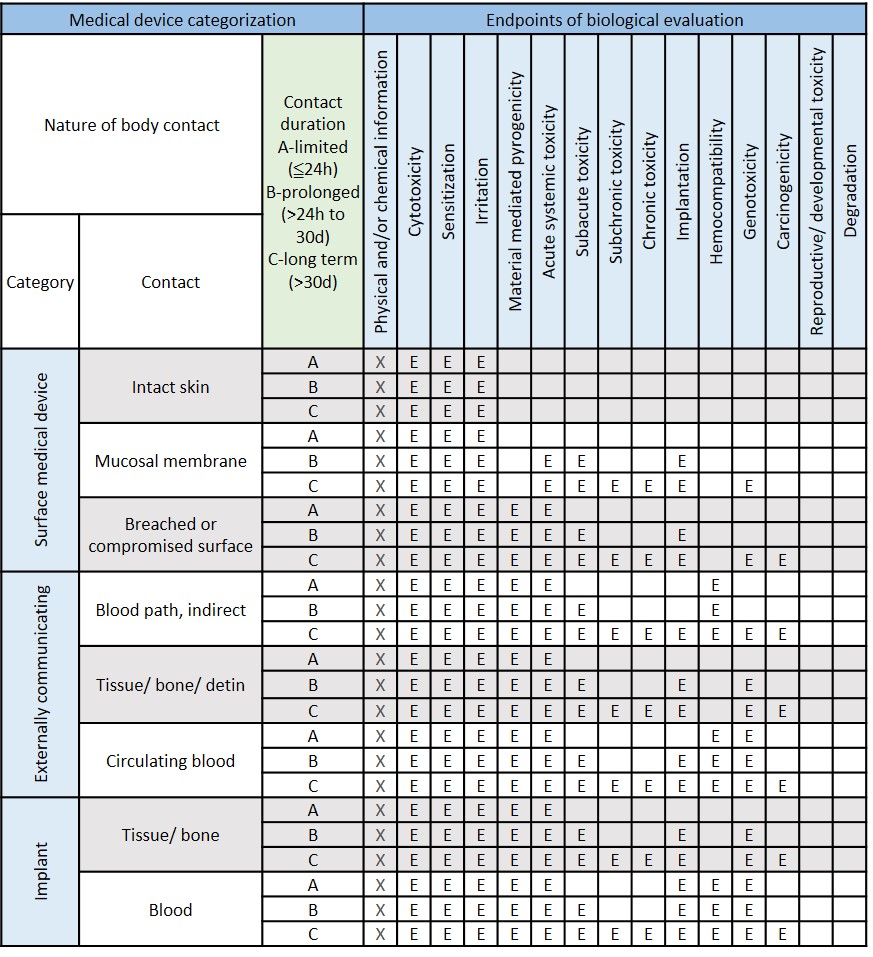
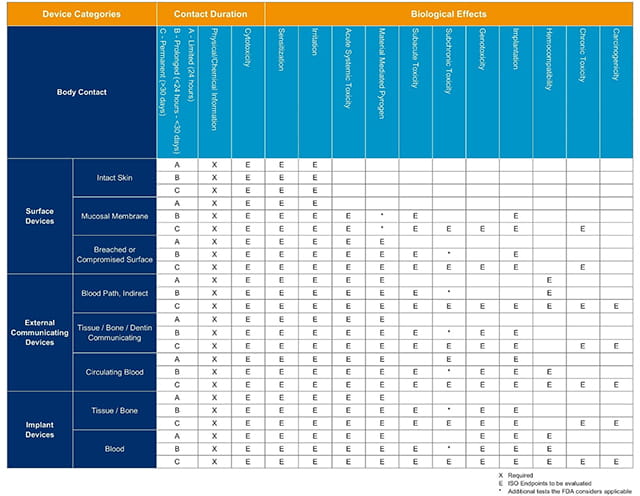
Table I from Towards a logic-based extension of a relational software tool for coherent technical documentation of medical devices | Semantic Scholar
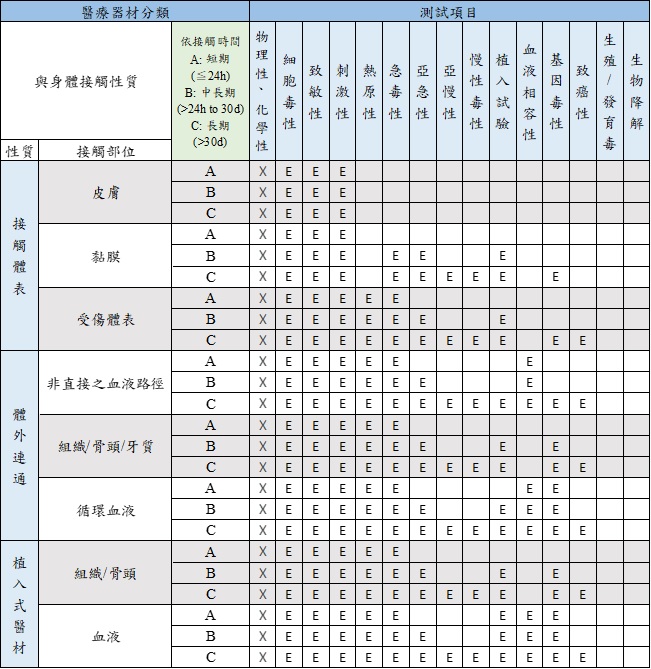
Biological Compatibility of Medical Service (ISO 10993) - Superlab CRO -health food, medical device, chemicals, pharmaceutical research (GLP&TAF accredited)